Phaseolus polytene chromosomes from embro suspensor
 |
 |
Coating of microscope glass slides
- rinse slides in a staining jar for 5 min in aceton and then air dry them
- apply a drop of 4 µl Poly-L-Lysin in the first third of the slide
- spread the drop using a second slide and let the coated slide air dry
Materials, chemicals and solutions
- 10 µl pipette
- staining jar, glass vessel
- aceton, technical
- Poly-L-Lysin (mw 100.000, Sigma, P-1274), 1 mg/ml in H20
Seed fixation
- harvest the pods (mostly mornings) in a big, lockable plastic bag and store them at +4°C for 1-3 days.
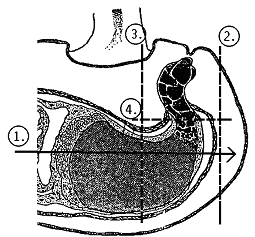
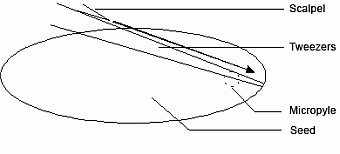
- open the pods along the joint using a sharp blade and dissect the tissue containing the embryo suspensor. For this, open the seed with a angular superficial cut (figure a) and cut out only the embroy tissue (figure b).
- store the disected tissues at +4°C for 3-7 days and change fixation solution after the first 24h
 |
 |
| Fig. a: surface section (longitudinal cut) | Fig. b: disection of the embryo tissue with suspensor below the microphyle (cross section) |
Preparation
- transfer fixed seeds/tissue from 70% Ethanol at - 20°C in 70% ethanol at room temperature and wash on slow rotation shaker for 5 min at min
- exchange ethanol with aqua dest. and wash 2x 10 min, like above. Note: Seeds/tissues should precipiate at the bottom within 20-30 min. If rotation is too fast, it takes more time for them to sink to the bottom!
- collect about 30 tissues in 10 ml MES-Sucrose buffer, rinse 3x und transfer them in plastic container with 15 ml 10% pektinase solution
- macerate tissues on a shaker at 120 rpm for 3 h at 37°C
- decant the pectinase solution and wash tissues 3x with MES-Sucrose buffer
- fix in 5 ml in solution of propanoic acid and lactic acid (1:1) for 3 h at room temperature
- rinse 3x in aqua dest. and leave at +4°C for 1-2 h
- transfer each tissue in some drops of 45% acetic acid onto a hollow slide
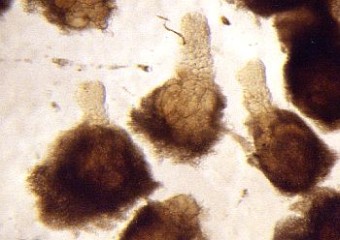
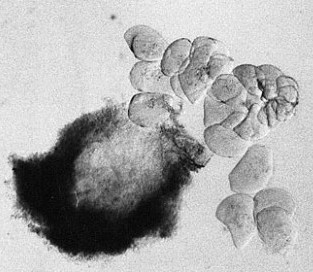
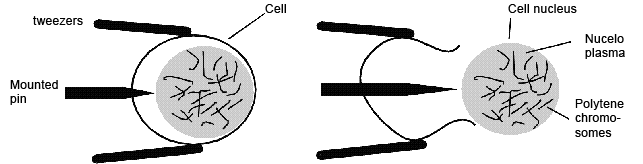
- separate the biggest cells of the suspensor with needles and tweezers (s. Foto RC2 und RC3)
- use only the biggest suspensor cells with very clear transparent cytoplasm (if one suspeonsor does not contain enough large cells, collect the biggest cells from multiple suspensors)
- take 8-10 large suspensor cells using a 20 µl pipette (with cutted tip) in 15 µl of 45% acetic acit and transfer onto poly-L-lysin coated slide. Alternatively for people with very cam hand:
- distribute single suspensor cells at sufficient distance from each other on the slide (cells must not overlap when the coverslip is landing with a smack, see next step)
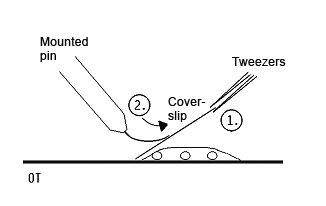
- take a coverslip and hold it angular against the slide with a tweezers(1); create some tention by pressing the needle against the cover slip (2)
- removing the tweezers suddenly, the coverslip is landing with a smack on the slide and squeezing the chromsomes out of the suspensor cells
- cover the cover slip with a filter paper (same size) and squash additionally by tapping the rounding back of a needle at right angle on the cover slip in order to seperate the chromosomes even more
- remove coverslip by dry-ice method of Conger and Fairchild (1953)
- dehydrate preparations by immersion in 99% ethanol and air-dry over night
- lable the position of the squashed cells at the border of the slide using a glass writing diamond!
 |
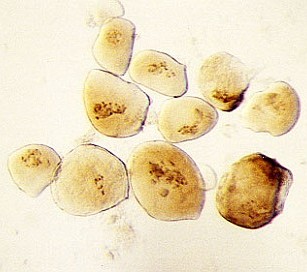 |
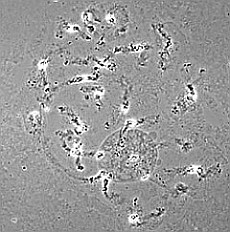
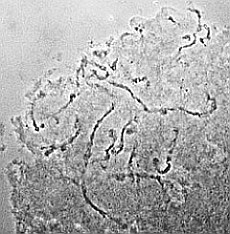
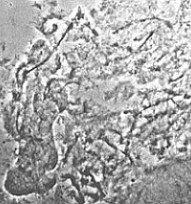
| RC2: Suspensor cells liberated from enclosing tissue | RC3: Big suspensor cells (10) in which the giant chromosomes appear as dark threads |
 |
 |
 |
good squashing |
medium |
insufficient |
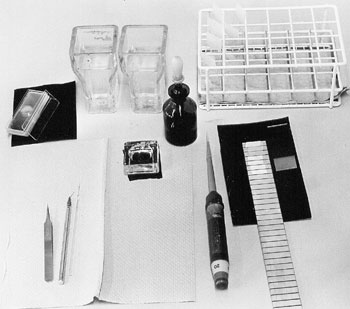
Material
 |
 |
| Instruments | Light microscopes |
- Scalpel, mounted pins, tweezers with fine tip, Pasteurpipette
- cover slips, 18x18mm
- microscope slides, microscope slides with 1 cavitiy
- filter paper
- cooling plate, razor blade, glass writing diamond
- plastic jars with with tight-closing lid (100 ml)
- 20µl-pipette, cutted 200µl-tips
Chemicals and solutions
- MES (2[N-Morpholino] ethanesulfonic acid) (Sigma, M-3023)
- sucrose (SERVA, 35580)
- pectinase from mould, 0.0095 units/mg (Fluka, 76290)
- acetic acid, 45%
- propionic acid, 99.5%
- DL-lactic acid
- methylated ethanol, 99%
- MES-Sucrose-Medium: 25 mM MES, pH 5.5 with 6% (w/v) sucrose

